Infusion Device Testing
There are a significant number of infusion devices (pumps and controllers) in hospitals and clinics worldwide used to control the delivery of a wide range of medications, nutrition and blood products. In anesthesia, the infusion device controls the delivery of narcotics, short-acting barbiturates and paralytics whose properties and actions on the physiology include analgesia, amnesia, paralysis and sedation. In the ICU, they are used for sedation and paralysis as well as infusing medications that help speed the healing of disease and chronic conditions.
Infusion devices have been the target of many clinical outcome investigations with human error, mechanical and electronic failure being the leading causes for failure. Incorrect readings from a poorly performing infusion pump can put patients at great health risk. Consequently, it is very important to test the performance of infusion devices to ensure they are performing in accordance with manufacturers’ specifications and clinicians’ expectations.
Pumps are equipped with sophisticated software and are being integrated into information networks and other clinical systems. It is important to understand the variety of mechanisms used in infusion pumps to control the flow rate and volume delivered. The most widely used mechanisms are roller (Fig. 1), linear peristaltics (Fig. 2) and syringe (Fig. 3). Each has different points of probable failure. The function of each has its own impact on the fluid flowing through the infusion tubing, which in turn, affects the measurement made to assess performance to the manufacturer’s specifications.

Infusion pumps require preventive maintenance at least once a year. Hospitals generally test infusion pumps at incoming inspection, scheduled PM and post repair; OEMs test infusion pumps for quality control. Preventive maintenance practices include verifying the pump is administering flow, volume and boluses accurately and occluding appropriately. These pumps are either tested based on protocols suggested by manufacturers or medical facilities, or according to the IEC 60601-2- 24 standard.
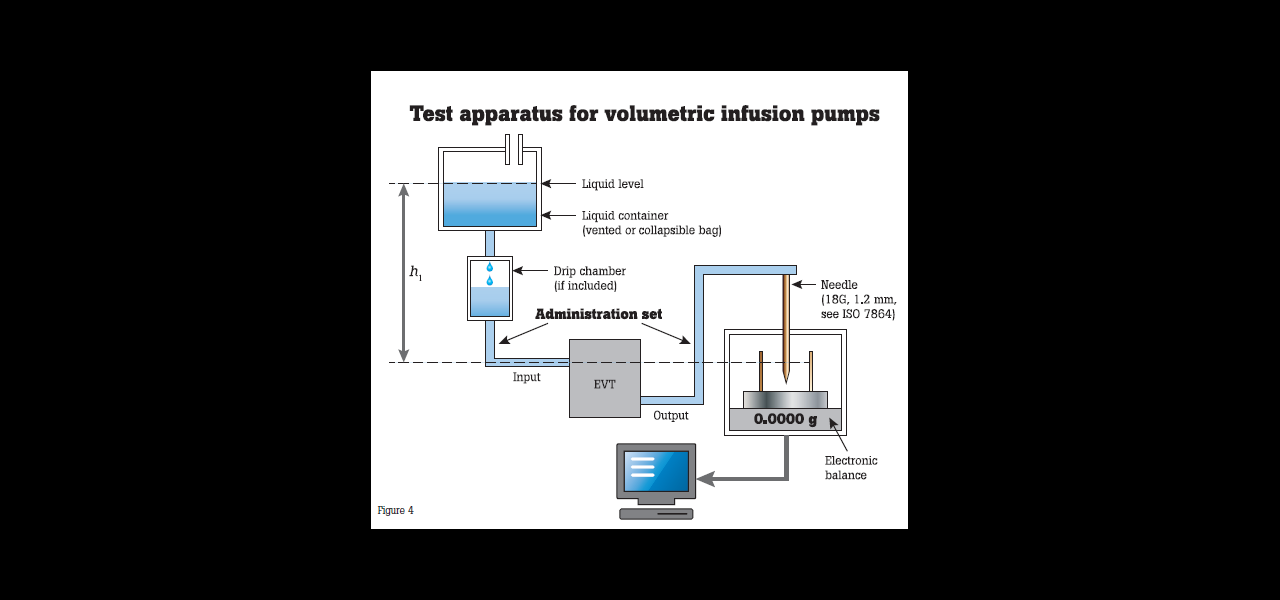
The IEC standard calls for volumetric infusion pump and volumetric infusion controller flow rates be assessed over a 120-minute test period using an intermediate flow rate of 25 mL/h. The infusion is delivered into a beaker and scale testing system.

Sources of error in the testing system suggested by the IEC standard include:
• Failure to zero the scale/balance with an empty, dry beaker.
• Miscalculation of the volume by weight (which is why the standard suggests an electronic scale/balance whose output is fed into a computer running the appropriate calculation to obtain the volume by weight).
• Loss of volume by evaporation (due to the length of the testing period). Note there is evidence in peer-reviewed articles that evaporation is negligible under normal ambient conditions.
Watch for the second blog regarding infusion pump manual and electronic testing.

